Accelerating the Commercialization of Smart Healthcare Products in Taiwan
Mission Statement
To accelerate the development and commercialization of smart healthcare solutions in Taiwan, the Ministry of Health and Welfare has established the Center for External AI Validation in Healthcare. Amid the global surge in smart healthcare innovation, access to high-quality clinical data remains a significant challenge. Many artificial intelligence (AI) models are trained on relatively small datasets—ranging from a few hundred to several thousand samples. As a result, these models often experience a notable drop in accuracy when applied in real-world clinical settings, limiting their practical utility.
To address the certification challenges faced by AI medical models in real-world clinical applications, the Ministry of Health and Welfare’s Department of Information Management, in collaboration with the Taiwan Food and Drug Administration (TFDA), launched Taiwan’s first-of-its-kind nationwide initiative: the Clinical AI Certification and Validation Center Program.
Through this program, five leading medical centers across the country were selected and awarded government funding to establish dedicated centers for external validation of clinical AI models. These centers enable AI developers and academic research teams to validate their models using larger-scale datasets that better reflect the characteristics of Taiwan’s population, thereby enhancing the quality, safety, and reliability of AI applications in healthcare.
Drawing from both international trends and local challenges in the development of smart healthcare, Dr. Chien-Chang Lee, MD, ScD, Director of the Department of Information Management, has developed an innovative framework for validation of clinical AI. This framework includes the implementation of federated learning platforms, the formation of hospital alliances spanning multiple healthcare systems, and the adoption of the international FHIR (Fast Healthcare Interoperability Resources) standard to develop interoperable, cross-institutional validation databases.
These external validation centers are open to global collaboration. AI product developers and research teams, both domestic and international, are encouraged to test and optimize their models on these trusted, third-party platforms to support the advancement of safe, effective, and locally relevant AI solutions in healthcare.
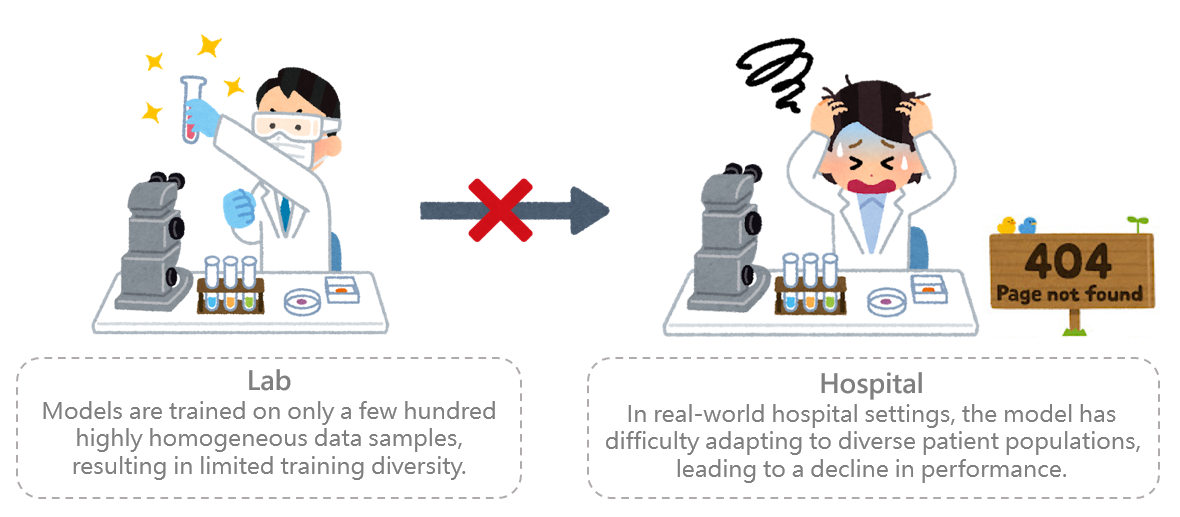
Figure 1. Challenges faced by AI medical models in real-world clinical applications
Core Strategies
1. Cross-System and Cross-Level Hospital Alliances
To ensure external validation reflects the diversity of patient populations across Taiwan, clinical AI models must be tested using data from hospitals of varying levels and healthcare systems. Validating models in diverse clinical settings enhances the assessment of diagnostic accuracy, cross-system adaptability, and performance consistency.
To support this effort, the Ministry of Health and Welfare’s Department of Information Management has established multiple Clinical AI Validation Centers. These centers form robust validation alliances by aggregating medical data from a network of hospitals, including regional hospitals and major medical centers. By leveraging pre-integrated, cross-system datasets, these centers streamline the validation process, enabling developers to obtain TFDA certification more efficiently and accelerating the commercialization timeline for AI products.
Each center is supported by a multidisciplinary team: clinical specialists provide domain-specific expertise; IT professionals facilitate model implementation and cross-institutional data integration; data scientists oversee data cleaning, standardization, analysis, federated learning, and model optimization; and epidemiologists or biostatisticians ensure the accuracy and rigor of validation performance metrics.
Figure 2. Cross-System and Cross-Level Hospital Alliance Framework
2. Federated Learning-Based Validation Architecture
The external validation framework supports two distinct approaches: centralized validation and federated learning-based validation. Centralized validation is recommended for heterogeneous data types, such as clinical case records or natural language processing applications, where centralized access improves analytic consistency. In contrast, federated learning is better suited for structured and more homogeneous data, —such as medical imaging and laboratory results, where model training can occur across institutions without the need to transfer raw data.
Federated learning is a decentralized machine learning methodology that enables multiple institutions to collaboratively train AI models while maintaining data securely on local servers. This approach enhances data privacy and security, ensures compliance with international regulations such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA), and increases model generalizability through exposure to diverse datasets. It also reduces the risk of data leakage during transmission and minimizes reliance on centralized high-performance computing infrastructure by utilizing local computational resources at participating institutions.
When validation results do not meet expected performance thresholds,, the validation centers provide not only a standardized report but also a curated set of de-identified data to assist developers in refining their models prior to resubmission. The outcomes of multi-center validation can be consolidated into formal validation reports for TFDA and applicant review. Furthermore, hospitals and developers are encouraged to collaborate on publishing high-quality scientific research based on the validation findings.
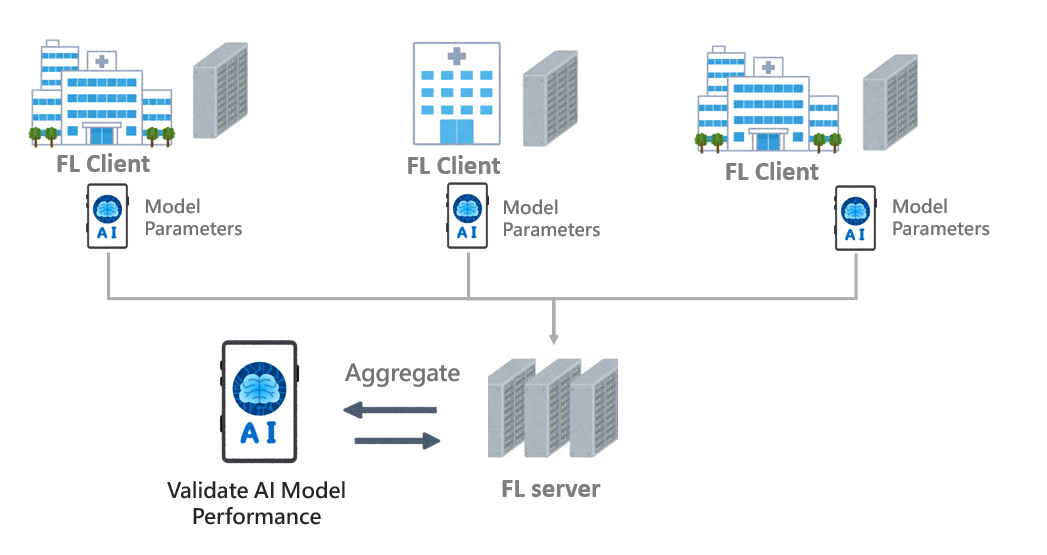
Figure 3. Federated Learning Architecture for External Clinical AI Validation
3. FHIR-Based Infrastructure for Cross-Hospital Validation Data Integration
A foundational component of this initiative is the construction of a cross-institutional clinical data infrastructure to support external validation. This effort begins with data standardization across participating hospitals. The project recommends adopting the Fast Healthcare Interoperability Resources (FHIR) standard—specifically, Taiwan’s national TW Core Implementation Guide—for consistent data integration.
All participating validation centers have implemented FHIR to connect hospital systems and build cross-hospital electronic health record (EHR) databases. This includes the development of terminology mapping dictionaries and automated tools for data cleaning and transformation, ensuring data are uniformly structured and aligned with international standards. This interoperable foundation supports future international collaborations and domestic data-sharing initiatives, significantly reducing integration time and improving overall data quality.
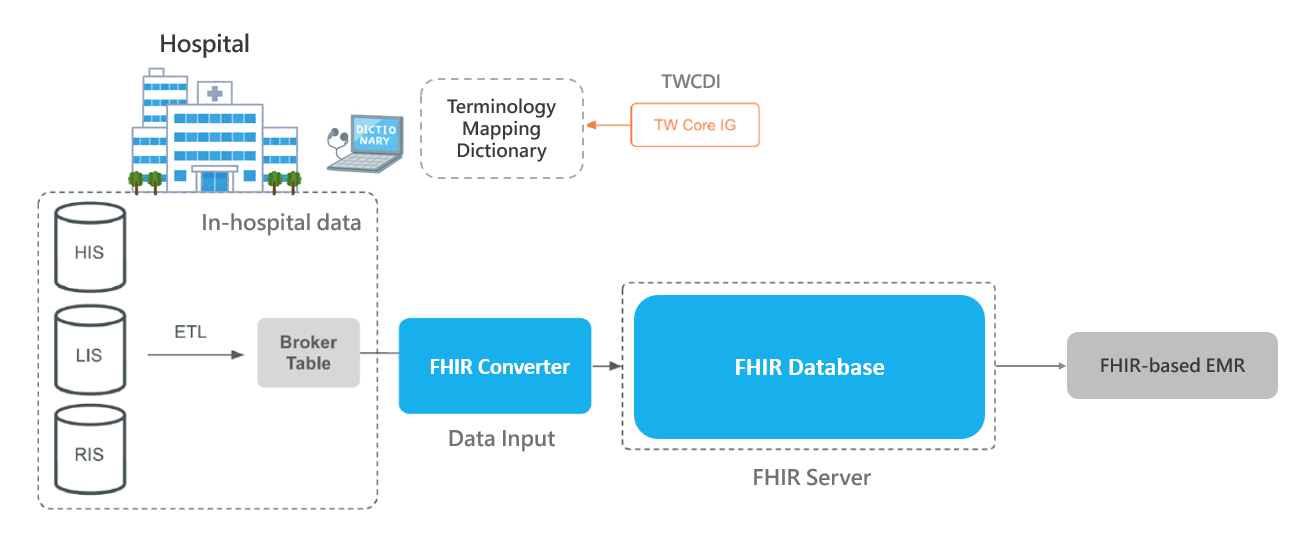
Figure 4. FHIR-Based Cross-Hospital EHR Integration Architecture
Future Outlook
Since mid-2024, the Ministry of Health and Welfare’s Department of Information Management has piloted the Clinical AI Certification and Validation Center initiative by providing funding to five hospitals. Within the first six months, these institutions successfully established cross-institutional medical data repositories based on the international FHIR standard and built AI model training platforms that support federated learning.
This innovative infrastructure lays the foundation for a national-scale, streamlined certification pathway for clinical AI, significantly shortening the time required to obtain regulatory approval from the Taiwan Food and Drug Administration (TFDA). It also promotes the secure and ethical reuse of electronic health record (EHR) data, maximizing the value of medical big data while upholding responsible data governance.
Taiwan’s external validation framework represents a first-of-its-kind system domestically and a pioneering model globally. In the coming year, the four hospitals that pass the final assessment will further expand their validation services, attracting a broader range of domestic and international smart healthcare companies and research institutions. With strong leadership from the government and active public-private collaboration, Taiwan is poised to become a global center of innovation in smart healthcare and clinical AI validation, opening new opportunities for the advancement of medical AI worldwide.
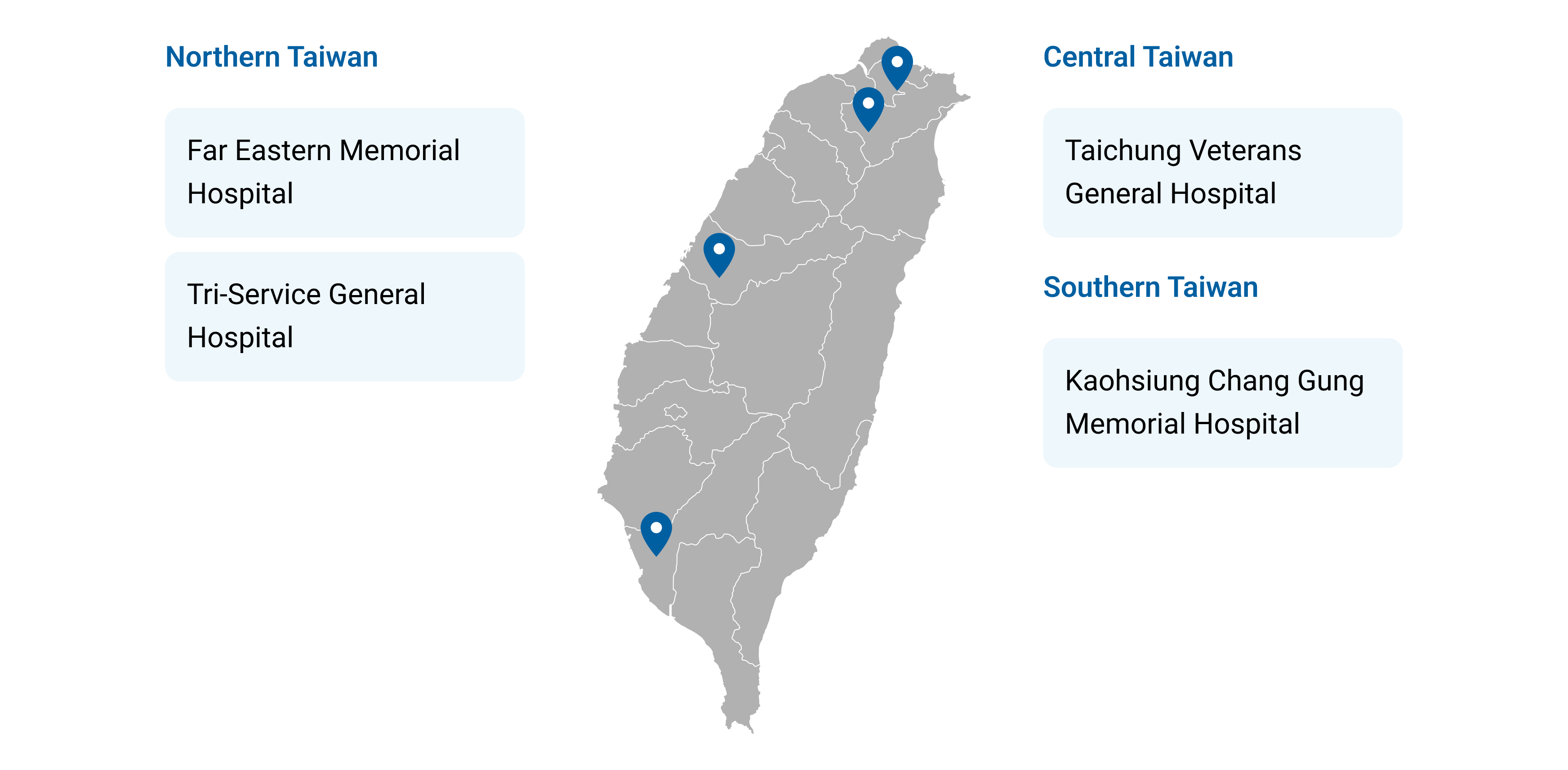
Figure 5. Taiwan’s Four Designated Demonstration Hospitals for Clinical AI Certification and Validation
【Northern Taiwan】
Far Eastern Memorial Hospital
Tri-Service General Hospital
【Central Taiwan】
Taichung Veterans General Hospital
【Southern Taiwan】
Kaohsiung Chang Gung Memorial Hospital
Project Team and Advisors
Project Initiator
Chien-Chang Lee, M.D., Sc.D.
Chief Information Officer (CIO) for the Taiwan Ministry of Health and Welfare (MOHW)
Professor of Emergency Medicine
Deputy Director of the Center of Intelligent Healthcare at National Taiwan University (NTU) Hospital
Acknowledgements
We sincerely acknowledge Shih-Ann Chen, MD, Honorary Superintendent of Taichung Veterans General Hospital and Convener of the Center for External AI Validation in Healthcare; Cheng-Kai Kao, MD, Associate Professor of Medicine, Chief Medical Information Officer, Medical Director of the Office of International Programs, and Medical Director of the Hospital at Home program at the University of Chicago Medicine (Chicago, IL, USA); Joshua Lin, MD, MPH, ScD, Associate Professor at Harvard Medical School and Executive Director of the Mass General Brigham (MGB) Center for Integrated Healthcare Data Research (Boston, MA, USA); and Leo Celi, MD, MS, MPH, Senior Research Scientist at the MIT Laboratory for Computational Physiology (Boston, MA, USA), for serving as external expert reviewers for External AI Validation in Healthcare.
Honorary Superintendent of Taichung Veterans General Hospital
Chief Medical Information Officer
Medical Director of the Office of International Programs
Medical Director of the Hospital at Home program at the University of Chicago Medicine, Chicago, IL, USA
Executive Director of the Mass General Brigham (MGB) Center for Integrated Healthcare Data Research, Boston, MA, USA

Senior Research Scientist at the Massachusetts Institute of Technology (MIT), Boston, MA, USA
Interntional Partner Matching Platform
 We support international organizations and innovators in identifying and connecting with the most appropriate partners in Taiwan, including the officially designated Centers for External AI Validation in Healthcare. To learn more, please click here.
We support international organizations and innovators in identifying and connecting with the most appropriate partners in Taiwan, including the officially designated Centers for External AI Validation in Healthcare. To learn more, please click here.